Every day, consumers select products off the shelf based on packaging claims such as “Fat-Free,” “Good Source of Protein,” and others. The packaging and labeling regulations are specific about how and when you can make a nutrient content claim. This blog will cover common claims and the criteria required to make those claims.
We will look at:
A note on RACC values: The available nutrient content claims are dependent on the latest RACC (Reference Amounts Customarily Consumed per eating occasion) values. You can find the list of RACC values here. Genesis R&D Foods uses the RACC value for your product to determine qualified claims.
A note on the CFR: The regulatory requirements for all available claims, including what criteria must be met for “good source,” “more,” “light,” and others, can be found in the CFR section 101.13 here.
Example: Low Sodium
From the CFR:
Because the use of a “free” or “low” claim before the name of a food implies that the food differs from other foods of the same type by virtue of its having a lower amount of the nutrient, only foods that have been specially processed, altered, formulated, or reformulated so as to lower the amount of the nutrient in the food, remove the nutrient from the food, or not include the nutrient in the food, may bear such a claim (e.g., “low sodium potato chips”).
To use the claim “Low Sodium,” on your packaging your food item must:
- Contain 140 mg or less of sodium per individual serving or per 100 g for meals and main dishes; and
- Have been formulated to lower the amount of sodium relative to foods of the same type; or
- Be a food that is inherently low in sodium; in which case, your claim must be phrased in terms that all foods of that type are low-sodium foods

Example 1: Potato Chips
- The RACC for one serving of potato chips is 30 g.
- Your analysis shows that there is 98 mg of sodium per serving of your potato chips, which meets the <140 mg qualifier.
- On average, potato chips have about 150-215 mg of sodium per serving, so your potato chips differ from other foods of the same type.
Your chips, therefore, qualify for a “low sodium” claim: “Low-sodium potato chips.”
Example 2: Plain White Rice
- The RACC for one serving of prepared white rice is 140 g.
- There is 15 mg of sodium per serving in your rice.
- Rice is an inherently low-sodium food, so the allowable claim/text is different.
Your rice can use the claim “Rice, a low-sodium food.”
Example: High in Vitamin C
“High” is used to indicate that one serving of a food item contains 20 percent or more of the Daily Value (DV) for a nutrient. For Vitamin C, the DV is 90 mg, so anything qualifying for a “high” will show 20% DV on the label.
Example: One serving of orange drink
- The RACC for one serving of your orange drink is 360 mL (12 fl oz)
- Your analysis shows that there is 72 mg of Vitamin C in one serving, which is 80% DV
- It, therefore, qualifies for a “high” claim.
In comparison, “Good Source of Vitamin C” means that the food contains 10 to 19% of the Daily Value of Vitamin C.
Example: Sugar Free
“Sugar free” on a package can be used if:
- There is less than 0.5 g of sugar per serving; and
- The item contains no ingredient that is a sugar product or generally understood to contain sugars;
- The package has a statement regarding the calorie content, such as “low calorie” or “not a reduced-calorie food.”

This differs from “No Added Sugar,” which means there was no sugar or sugar-containing ingredients added during processing.
BUT…
There’s one more consideration here, too. If you use one of the claims listed above, but your food had exceeded DV threshold levels for fat, cholesterol, saturated fat or sodium, you have to use the disclosure “See nutrition panel for {nutrient} content.”
Example: Your item is “sugar free” but it contains more than 13 g of fat per serving, which exceeds the DV. You can use the “Sugar Free” claim, but the package must also say “See nutrition facts for fat content.”
This all sounds complicated and time-consuming. And it can be. The good news, however, is that our program – Genesis R&D Food Formulation & Labeling Software – has the claims regulations built in AND will tell you if your Recipe qualifies for any claims. With the click of a button, you can know if your recipe is “low in Sodium” or “a good source of Vitamin D,” etc.
The following instructions will use the Recipe “Sample Fruit Salad” as an example.
Please note: Make sure you have selected the correct regulation (Edit Label > General) first. If you want your claims to correspond to the 2016 labels and not the 1990 labels, you have to have the 2016 regulation selected.
- Open a Recipe
- Select Claims from the Recipe ribbon. If this is the first time using the claims feature for this Recipe, you will get this message:
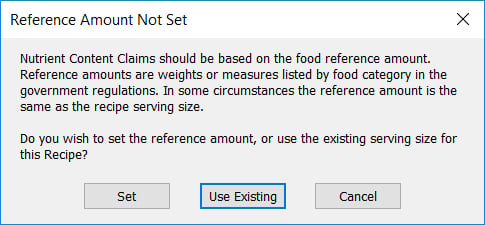
- To make sure Genesis R&D is correctly evaluating your product for the qualified claims, you must use the RACC for your product. If the RACC amount is the same as the Recipe serving size, select Use Existing. If not, select Set and enter the correct reference amount.
- On this screen, any nutrient that qualifies for a packaging claim will be indicated with a green checkmark.
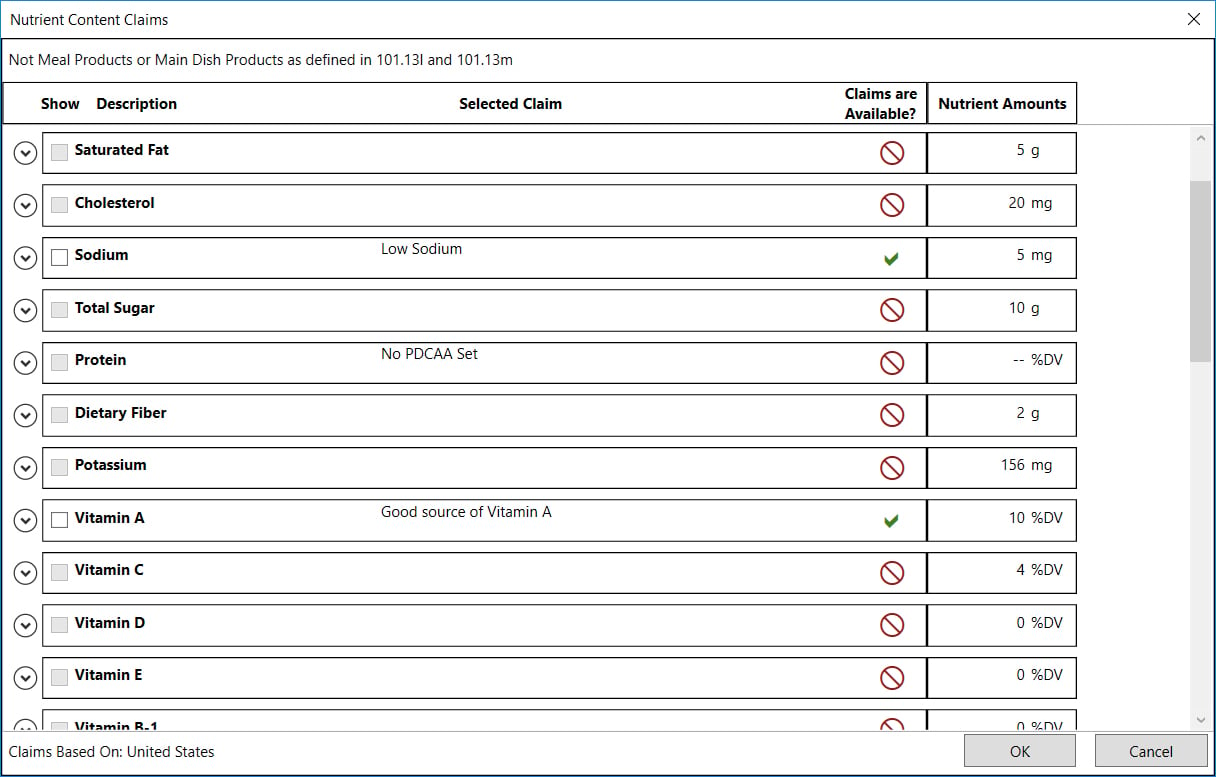
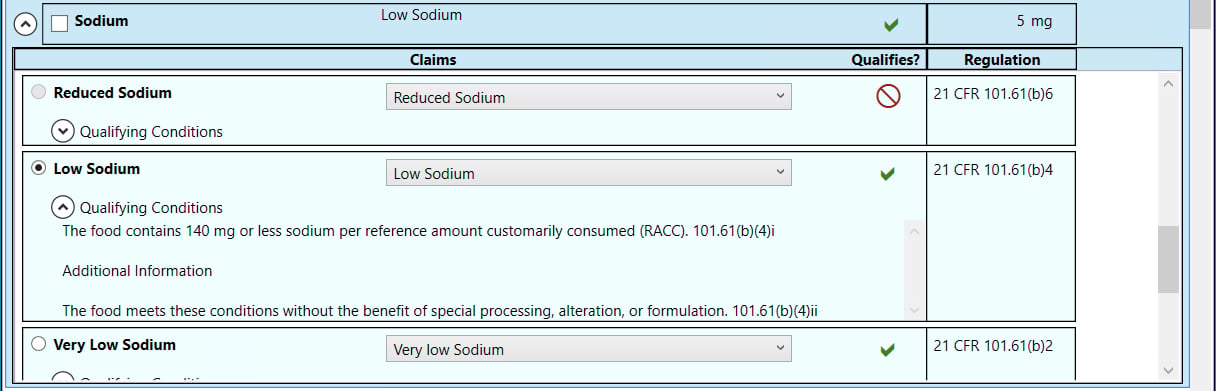
- When you expand the nutrient, as shown here for Low Sodium, you can see the specific claim, its qualifying condition and where you can find more information in the CFR.
- And by expanding the low sodium menu, you can see what specific wording you have to choose from.
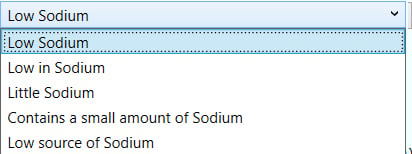
- When you check the claim box, that claim will appear on your Label view page.
Note: Although Genesis R&D makes it easy to quickly identify what claims your food qualifies for, it is a best practice to double-check with the CFR and consider all of the required conditions for making claims on your product packaging.
Watch this tutorial to learn how the Nutrient Content Claims Feature in Genesis R&D Foods works.
Other posts you might be interested in
View All Posts
Food Labeling
10 min read
| January 1, 2022
Using the Attributes Feature in Genesis R&D for Tracking BE Material and other Recipe Characteristics
Read More
Product Formulation
15 min read
| April 18, 2019
How to Create an FDA-Compliant Nutrition Facts Label
Read More
Product Formulation
9 min read
| May 10, 2023

