Consumers are going to start seeing results of the decades-long debate over GMO labeling with the finalization of rules establishing the national mandatory bioengineered (BE) food disclosure standard. USDA published the rules on Dec. 21, 2018, and regulated entities have until January 2022 to comply.
The new regulations — called The National Bioengineered Food Disclosure Standard (NBFDS) — conclude a July 2016 directive from Congress to develop GMO, or bioengineered, labeling standards, including a definition of bioengineered and which food producers and/or distributors would fall under the law.
Note: The NBFDS adopted “bioengineered foods” as the preferred nomenclature, essentially replacing “GMO.”
Overview of NBFDS
The USDA’s NBFDS (“the Standard”) establishes:
- What foods are considered bioengineered
- What food-related entities must disclose BE information
- When disclosure must be implemented
- What terms and images can be used for disclosure
What foods does the Standard apply to?
Any food product (including chewing gum and some alcoholic beverages) intended for human consumption and that:
contain detectable genetic material that has been modified through in vitro recombinant deoxyribonucleic acid (rDNA) techniques and for which the modification could not otherwise be obtained through conventional breeding or found in nature
are considered to be bioengineered foods and therefore must display a disclosure statement or image. This pertains to both raw agricultural commodities and processed or multi-ingredient foods (with some exceptions). Food served in restaurants, highly refined foods with undetectable modified genetic material, and some meat products are exempt, for example. A list of foods commonly found in bioengineered form can be found on the USDA’s website.
Please note: Many supplement and nutritional products fall under the “Food” umbrella and must be considered as such with regard to BE disclosure.
Who must comply with NBFDS?
Regulated entities include food and dietary supplement manufacturers, food and dietary supplement importers, and other food retailers, including those that package and sell food in bulk. Small food manufacturers (those with annual receipts of at least $2.5 million but less than $10 million) have a later implementation date and very small food manufacturers (those with annual receipts of less than $2.5 million) are exempt.
Also exempt are restaurants and similar retail food establishments (cafeterias, food trucks, airplanes, etc.).
When do regulated entities need to comply?
- The standard implementation date is Jan. 1, 2020, for most manufacturers and Jan. 1, 2021, for small food manufacturers.
- Voluntary compliance ends Dec. 31, 2021.
- The final mandatory compliance is Jan. 1, 2022.
Regulated entities should start the compliance process no later than the standard implementation dates by identifying the foods that will need to bear a BE disclosure, compiling and reviewing the records necessary to meet the record-keeping requirements, and deciding which type of BE disclosure they will use on their products.
 Display requirements for BE foods
Display requirements for BE foods
The Standard offers a variety of disclosure methods to help regulated entities more easily comply.
The disclosure must be on the food and supplement package in either:
- The information panel adjacent to the manufacturer/distributor information, or
- The principal display panel.
If there is insufficient space on either of those panels, then on any other panel likely to be seen by a consumer under ordinary shopping conditions.
There are four options for making a disclosure:
- On-package text, e.g. “Bioengineered Food,” or “Contains a Bioengineered Food Ingredient.”
- USDA approved symbol.
- Electronic or digital disclosure, such as a QR code. It must include instructions to “Scan here for more food information” or similar language and include a phone number.
- Text message disclosure: “Text [command word] to [number] for bioengineered food information.”
Additional Consideration
The NBFDS preempts any state or local bioengineering labeling or disclosure standards.
How does Genesis R&D help with BE disclosure?
Within the Genesis R&D Supplements program, there are options for helping you track and report any bioengineered ingredients and formulas. We suggest using Groups and the Percent Organic option.
Groups
The process works like this:
- Create a group.
- Assign your ingredients to the Group.
- Filter your searches by inclusion or exclusion of the Groups.
In the example below, the Green Tea ingredient is open. Select “Certified Non-BE” from the available groups (or, if it has not yet been created, type in “Certified Non-BE” and create the Group.)
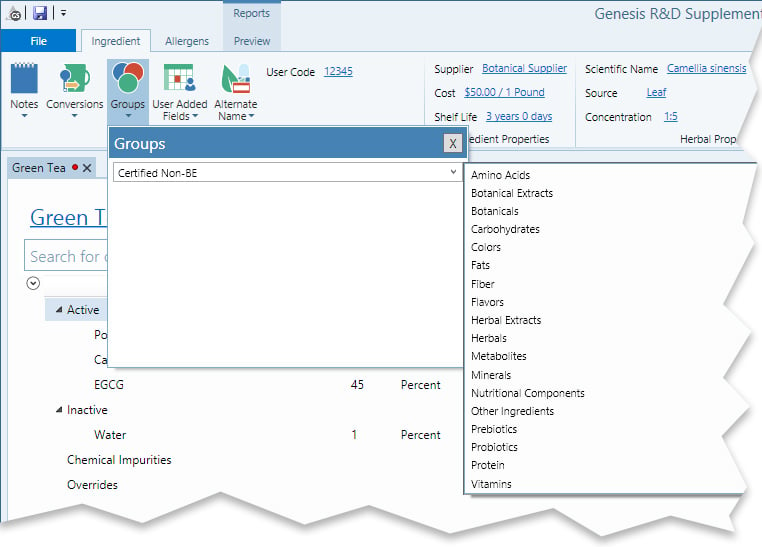
Now, when you create a new formula, you can filter ingredients either in the search or in the Formula creation window by inclusion in the Certified Non-BE Group, like so:
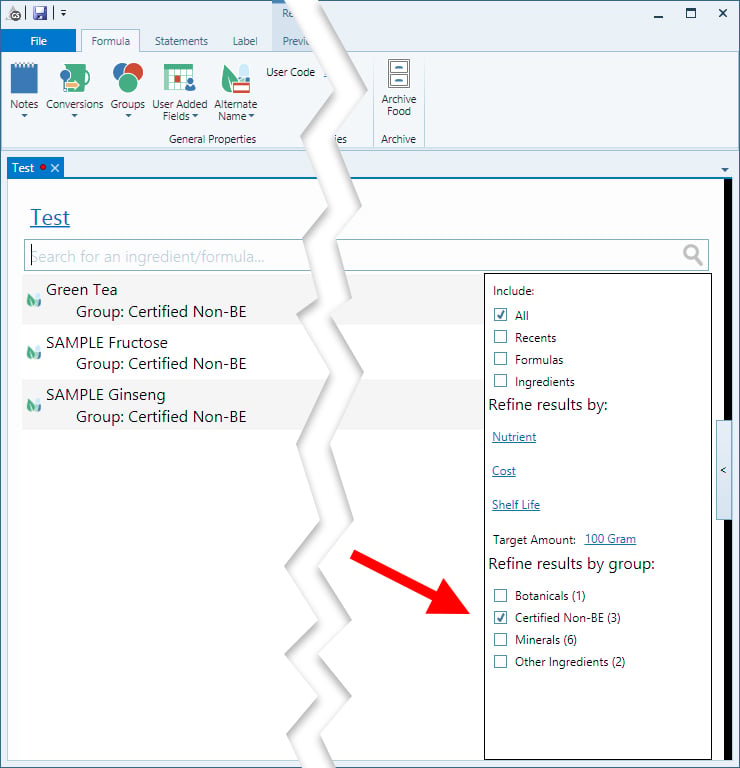
Then, you can create a new formula Group for all formulas that contain only certified non-BE ingredients.
In the same manner, you could create other Groups for similar purposes. Perhaps “Under Review” or “Pending Certification” or “Needs BE seal.” Whatever is most helpful.
Reports
Any Groups an ingredient or formula belongs to will show up on that record’s report.
Using Percent Organic to Identify BE Status
Some food retail businesses and certification bodies (Oregon Tilth is one example) use the “Organic” umbrella to include any non-BE items. If this is true for you, the Organic Properties feature may be useful.
You can enter an organic percentage at the Ingredient level, and set target organic percent amounts at the Formula level. The program will automatically calculate the total Organic Percentage and indicate that at the bottom of your screen.
If the target amount is met or exceeded, the percent will appear in green.

If not, the percent will be shown in red.

Use this data to help you decide whether to reformulate your food or to label it as bioengineered.
Other posts you might be interested in
View All Posts
Food Labeling
11 min read
| May 25, 2021
Using Genesis R&D to Comply with the UK’s Food Information Allergen Declaration Amendment
Read More
Product Formulation
12 min read
| June 21, 2023
How to Create Accurate Allergen Declarations and Ingredient Statements with Genesis Foods
Read More
Food Labeling
18 min read
| July 13, 2022

