The FDA regulates most packaged foods sold in the United States and has specific requirements for what elements a package must contain (e.g. a Nutrition Facts panel, ingredient statement, etc.). In order to sell your food products, you must comply with the FDA’s packaging laws unless your operation is exempt.
The regulations are a little complicated, but this blog should help offer clarity. We will cover the basics of packaging regulations, including:
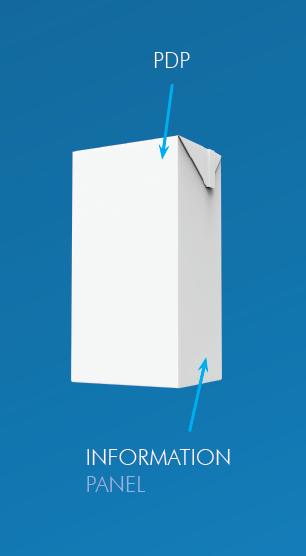
Before we discuss placement, it’s important to understand what each area is called and where it is on the package.
This is the area most likely to be seen by a buyer at the time of purchase. For a rectangular container like a cereal box, the PDP area is the product of the height times the width. For a cylindrical container like a can, the PDP area is 40 percent of the product of the height times the circumference.
The information panel is the panel (or space, if the package is cylindrical) immediately to the right of the PDP.
The Statement of Identity is the legal name of the food (example: Nilla Wafers), the common name of the food (example: peanut butter), or, when the other two are not appropriate, a description of the food (example: whole green peas). This is not the same as the brand name (example: Kellogg’s).
Placement: The Statement of Identity must be placed on the PDP as one of the primary art elements. The font type height should be, at a minimum, half the size of the largest font on the package.
Net quantity is simply the amount of food in the package, shown as a weight, fluid measure, or the number of items.
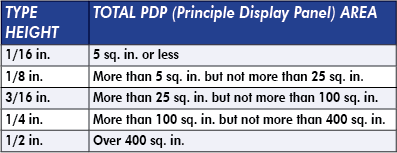
Placement: This is placed in the bottom 30 percent of the PDP in a type height determined by total PDP area, per this chart:
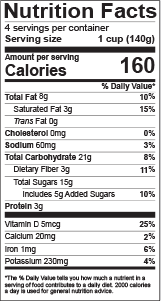
The Nutrition Facts Label is used to communicate important information about the food consumers eat. The FDA also governs what label format to use on your product based on package size and contents.
The Nutrition Facts Label must show:
- Serving size (Consult the RACC to determine this)
- Household measure/common household unit
- Servings per container
- Mandatory nutrients (total calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, potassium)
Placement: In general, place the Nutrition Facts Label on the PDP or the Information Panel, near the ingredient statement.
You must display the ingredient statement on the same panel as the manufacturer’s information. The ingredients are listed in descending order of weight in a type at least 1/16” tall and easy to read.
Placement: In general, place the Ingredient Statement on the PDP or the Information Panel, near the Nutrition Facts label.
The Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) mandates that packaged food items must declare, in plain language, the presence of any major food allergens (Milk, Egg, Fish, Crustacean shellfish, Tree nuts, Wheat, Peanuts, Soybeans, Sesame) on the product packaging.
Placement: In general, place the Allergen Declaration on the PDP or the Information, near the Nutrition Facts Label.
Allergens may appear either:
- In a parenthetical directly after the name of the ingredient within the ingredient statement. Example: Peanut butter (peanuts), casein (milk), spice (sesame)…
OR
- In a separate “Contains” statement. Example: Contains peanuts, milk, and sesame.
The food package must show the name of the manufacturer (or packer/distributor, accompanied by a qualifying phrase that states the firm’s relationship to the product, e.g., “manufactured for” or “distributed by”) and the full street address.
Placement: Most often, this goes on the Information Panel, but, again can appear on the PDP.
A Nutrient Content Claim is any statement regarding a nutrient level in your food (examples: “low fat,” “high fiber,” “sugar-free”).

Regulations determine what claims are valid.
Claims can be displayed on the PDP, Information Panel or anywhere else on the package, in a type size that can’t be larger than twice the size as the font used for the Statement of Identity.
Important: If you choose to use a Nutrient Content Claim on your package, you must have a Nutrition Facts Panel showing that nutrient and its value.
No government regulatory agency requires that your food package have a barcode. Most retail establishments, however, will. The barcode must be placed in a manner where it doesn’t interfere with the required elements.
Some states require dates on some foods. You will need to check into the specific regulations for your state and food.
As far as placement on the package, the date cannot interfere with required labeling elements and must show the month, day, and year immediately adjacent to an explanatory phrase (“best before,” “sell by,” etc.)
Learn More
eBook: Food Labeling 101
For a more extensive and printable version of this blog, download our ebook: Food Labeling 101.
FDA eCFR Resources
For more information on the regulations, please refer to the Code of Federal Regulations. Use the drop-down menu to select Title 21 and click on 100-169 under Browse Parts.
Tag(s):
Food Labeling
Other posts you might be interested in
View All Posts
Food Labeling
12 min read
| January 19, 2024
Health Canada’s Front-of-Package Labelling Updates
Read More
Food Labeling
30 min read
| November 8, 2020
Creating Mexico Front-of-Package Warning Seals and Statements
Read More
Food Labeling
6 min read
| September 14, 2020