GS1 US, along with a diverse FSMA 204 workgroup that includes Trustwell experts, has created a new industry guideline titled: “Retail Grocery and Foodservice Application of GS1 System of Standards to Support FSMA 204.”
GS1 US, the not-for-profit organization that develops and maintains the global system of standards for barcodes, has created a valuable new resource for food supply chain stakeholders. The guideline includes supply chain diagrams and data examples, using GS1 standards.
Some Background on GS1 US and FSMA 204
In November 2022, the Food and Drug Administration (FDA) published their final ruling for Section 204 of the Food Safety Modernization Act (FSMA 204). The finalized ruling included a list of foods that will require additional traceability recordkeeping, as well as the data elements that businesses will need to collect to prepare for compliance by the January 20, 2026 deadline.
GS1 standards, from barcoding to EDI, are widely used across food supply chains. While the FDA does not prescribe any named standard or approach, they do mention that standards and industry guidelines such as GS1, PTI, and GDST are vehicles that could be utilized to meet the recordkeeping requirements of FSMA 204. The hope is that through standardized identification of product and lot information, the food supply chain will be able to act more quickly when product recalls occur to trace, remove, and understand the source of contaminated products.
As such, GS1 US has stepped up to help businesses understand how to use standards as they adapt to FSMA 204’s requirements.
Retail Grocery and Foodservice Application Resource
The GS1 US Implementation Guide was developed alongside stakeholders, including our very own Julie McGill, VP of Supply Chain Strategy and Insights at Trustwell. The workgroup identified scenarios where GS1 standards could apply to FSMA 204 compliance, and how tools such as barcoding can support the necessary data capture and make the transition smoother.
Applying GS1 Standards to FSMA 204 Recordkeeping Data
The FSMA 204 final ruling requires the collection of traceability lot codes (TLCs), critical tracking events (CTEs), and key data elements (KDEs) relevant to the product’s stage in the supply chain. Data attributes are defined in the final rule and are intended to help the FDA during an investigation. The standardized data can include things like a product and brand name, species and variety identifiers, contact information for farms or manufacturers, point of contact for an event, and more (see image below).
Specifically in the final rule, the FDA encourages food companies to “use any tools that will improve a firm's procedures for traceability and support the maintenance and sharing of the required traceability records under the final rule.” GS1 standards can be applied to the collection of this data in a couple of ways:
- Product data: To capture product data, companies can use a Global Trade Item Number (GTIN), a GS1 standard for identifying products, as an additional identifier, along with other key attributes that further define the product itself.
- Location data: To identify physical locations, companies can use a Global Location Number (GLN), along with other key attributes that help define the location or facility.
- Event data: This is the dynamic data related to the activity or event that has occurred, as defined in the rule, such as shipping, receiving, harvesting, etc.
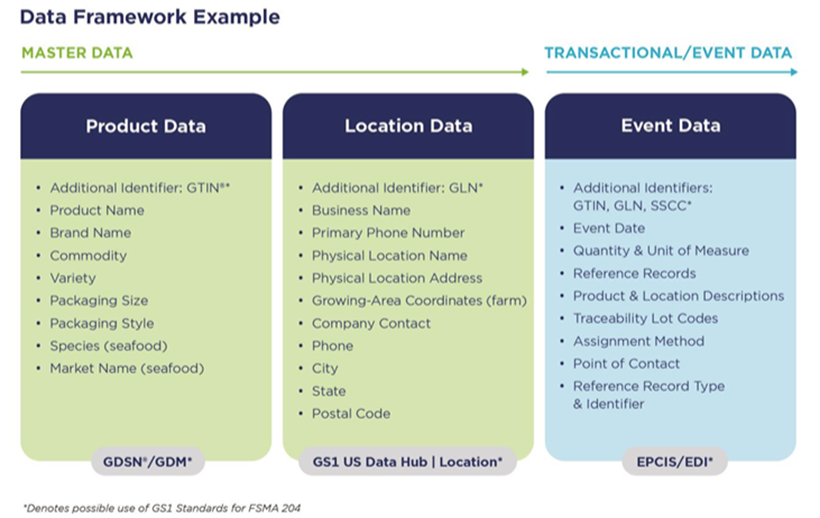
(Image courtesy of GS1 US)
How Businesses Can Prepare for FSMA 204 Compliance Today
The guidance provided by GS1 US is voluntary and not mandatory for FSMA 204 compliance. However, companies that have already adapted GS1 standards for traceability may find that they’re a step ahead when it comes to achieving compliance. GS1 standards provide a standardized approach to product, location, and event data. Adopting a shared approach enables connectivity, interoperability, and full chain traceability and visibility.
The shared language between supply chain partners is a vital component of the FDA’s New Era of Smarter Food Safety blueprint. So, businesses that use GS1 adapted systems to track inventory levels, identify products and locations, or capture traceability data are just one step closer to achieving the new recordkeeping requirements.
Choosing the Right Technology Partner for GS1 and FSMA 204 Success
As an established Solution Partner with GS1 US, Trustwell’s FoodLogiQ platform helps companies collect, monitor, maintain, and share required traceability data for FSMA 204. Beyond 204 compliance, FoodLogiQ provides supply chain visibility and transparency for your products at any stage in their journey from farm to plate.
To learn more about how your business can prepare for FSMA 204 today, contact our team to schedule a free FSMA 204 consult, or a more in-depth overview of the ruling with our FSMA 204 Professional Services. Our experts will provide an overview of the rule, discuss your affected products, and share how Trustwell's FoodLogiQ Traceability platform can help support your business’ compliance needs.
Tag(s):
Other posts you might be interested in
View All Posts.png?length=360&name=MicrosoftTeams-image%20(20).png)
Supplier Compliance
3 min read
| June 2, 2023
Find Trustwell at GS1 Connect: Booth #21 and More!
Read More
Food Safety
6 min read
| October 14, 2022
Modern Traceability Recordkeeping, FSMA 204, and the Supply Chain
Read More.png?length=360&name=BlogPostFeaturedImage%20(1).png)
Food Industry
4 min read
| February 24, 2022

